The movie Thor came out a few months ago, featuring some killer CGI, really nice nebula art, and one line that bothered every right-thinking astrophysicist: that Thor’s mystical hammer Mjolnir was “forged in the heart of a dying star.” It’s true, Mjolnir was forged in the heart of a dying star – just like every other hammer on the planet Earth, along with anything else made from iron or steel. You’ve probably heard the old quote from Cosmos that “we are all made of stardust.” But that’s not the whole story. How that dust gets made is an intricate tale that spans a wide range of stellar processes and masses. This is the field of nucleosynthesis, the making of the chemical elements, and it is what allows us to make the simple statement: toothpaste comes from neutrinos.
In the beginning, there was the Big Bang. As the fiery new universe cooled, energy organized itself into progressively more complex material forms: first quarks, then hadrons, then nuclei. But so-called Big Bang nucleosynthesis halts at lithium. The rapid expansion of the early universe, combined with the instability of all nuclear isotopes with mass 5, prevents it from proceeding any further. We must look elsewhere for the rest of the periodic table. In 1957, Margaret and Geoffrey Burbidge along with William Fowler and Fred Hoyle published the landmark paper Synthesis of the Elements in Stars, more commonly called “B-squared-F-H.” B²FH proposed that heavier elements – what astronomers call metals, i.e., anything that’s not hydrogen or helium – were fused in stellar cores.
The temperatures, densities, and pressures at which these fusion processes occur, as well as the mass ratios of the stellar populations that form them, all leave their own distinct fingerprints on the pattern of cosmic abundances. We can use those traces to learn about the evolution of the universe and of the stellar population by tracing the metal content of stars throughout cosmic time, as well as by matching the relative abundances of the elements produced by simulations against the observed values. We benchmark these values by the solar abundance set, or the relative amounts of the naturally occurring isotopes present in the sun (as measured by spectroscopy) and the presolar nebula (as measured by meteorites).
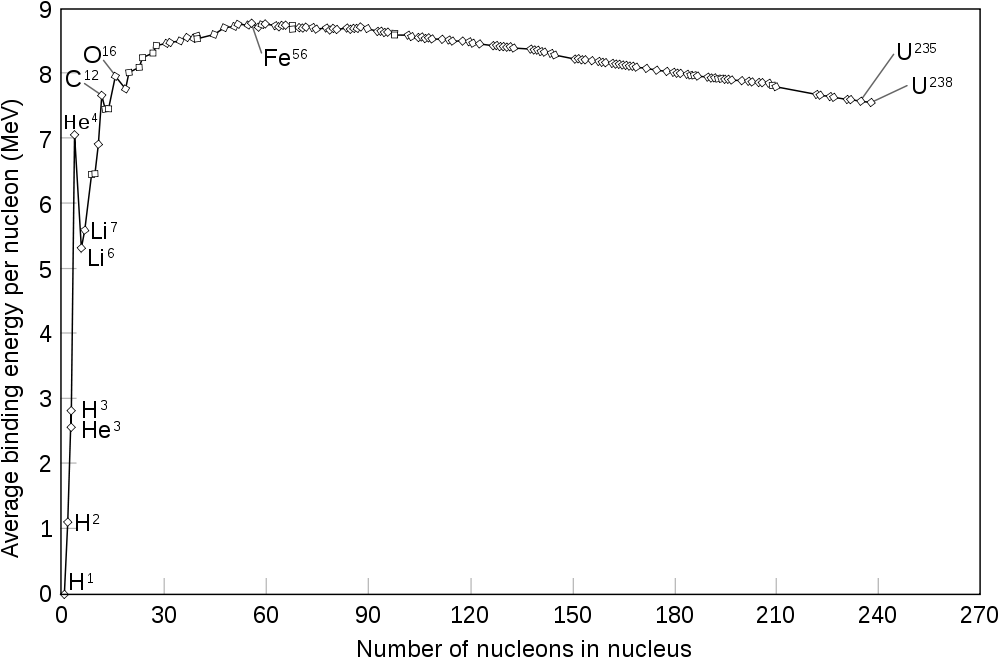
The curve of nuclear binding energy vs. increasing nucleon number A. The curve rises steeply to a maximum around iron-56.
The fact that heavier elements are made in stellar cores is probably not news to most of our readers. But the tale of how all the elements are made is a remarkably complex one. The one fundamental curve that explains much of stellar nucleosynthesis is the curve of nuclear binding energy, seen to the right. This curve shows how tightly bound an atomic nucleus is, i.e. how much it does or does not want to split apart. The higher up a nucleus is on the graph, the more tightly bound it is. Moving upwards on the graph – say, through fusion from hydrogen to helium, or through fission from uranium to krypton – yields energy. Moving downwards requires energy. The notable thing about this curve is that it has a maximum: there is an isotope in the middle of the periodic table that is the most tightly bound. Technically, the question of which isotope is at that peak depends on the environment, as it changes slightly with the electron fraction, but it is usually either iron or nickel. This creates the iron peak, the group of tightly-bound elements around iron. Simple fusion in stellar cores cannot proceed beyond this point. We need additional processes to create elements heavier than this.
While chemists are content with the simple periodic table you saw in high school, astronomers and nuclear physicists like to put things a bit differently. This is the periodic table of nuclear isotopes, color-coded by half-life (darker = more stable). The dark line running through the center is called the valley of beta stability, the region where elements are proof against beta decay and hence relatively long-lived (they may still decay, but with half-lives longer than the observed universe).
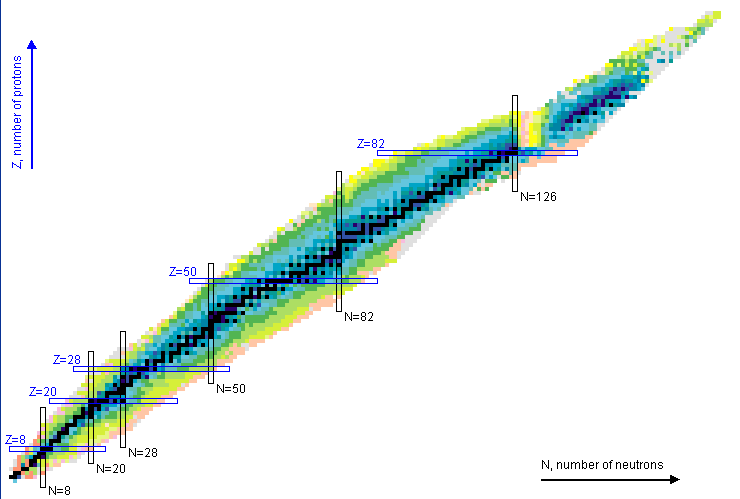
The chart of nuclides, showing all known isotopes. The horizontal direction is increasing neutron number N, whereas the vertical direction is increasing proton number Z. Each square is an isotope, color-coded by its radioactive half-life (darker = more stable). The open rectangles at certain numbers show the magic numbers of stability where nuclear shells are closed and nuclei tend to be stable.
There are three main types of isotopes to be made: the neutron-rich isotopes, the proton-rich isotopes (or p-nuclei), and the isotopes with roughly equal numbers of protons and neutrons. Neutron-rich nuclei are primarily created by the r process; p-nuclei are primarily created by the combined efforts of the p process and the gamma or photodisintegration process; and all the elements in the middle are built up by the s process. There are also a set of extra processes, such as np, rp, v, and vp, that represent variants on these or fill in oddball elements that would otherwise be skipped.
The s process (s for “slow”) is the reliable workhorse of post-iron nucleosynthesis, and was proposed as far back at the original B²FH paper. The name comes from the fact that neutrons are added much slower than the beta decay timescale. A seed nucleus of an iron peak element picks up free neutrons – heading out to the right of that graph – until it becomes unstable. It then decays back into the valley of beta stability, increasing its proton number, allowing it to pick up more neutrons and begin the cycle again, zigzagging its way up through the elements. The s process can’t stray from the valley of beta stability, and it builds on previous s process nuclei to produce higher and higher A elements. This process produces basically all isotopes with A > 90 in the valley of beta stability up through lead-210/bismuth-208, where it gets stuck in a loop of alpha-decay.
The s process occurs in massive stars on its own, in the later stages of nuclear fusion. However, because the s process builds on its own products, we actually require a distribution of exposure strengths – the ratio of free neutrons to seed nuclei – to produce the whole solar abundance set, because the lighter elements must be consumed to produce the heavier elements. Thus, the main component of the s process is actually produced in asymptotic giant branch (AGB) stars, during helium shell flashes. As helium burns in a shell around the core, it makes some amount of s process elements; when the shell burns out, the fused ashes are swept down into the rest of the star, and some portion of them are recycled into the next helium shell to receive another exposure. This produces a distribution of exposure strengths, as some percentage of each batch is recycled into the next one.
The r process is probably the most well-known of the nucleosynthetic processes. The r stands for “rapid,” and it occurs when a nucleus with equal numbers of neutrons and protons suddenly picks up a large number of neutrons (i.e. heading very far to the left on our chart of nuclides). Neutrons are added one by one – but, importantly, in rapid succession, so that the nucleus does not have time to decay radioactively – until it runs up against the neutron drip line, where the energetic balance favors losing extra neutrons as soon as they are gained. Once the flux of neutrons drops, the nucleus will decay, trading neutrons for protons, until it reaches the outskirts of the valley of beta stability and stabilizes as a neutron-rich, long-lived isotope. The r process stalls out when it reaches A = 270, as the nuclei there begin to fission spontaneously.
Up until recently we thought we had the r process figured out as occurring in core-collapse supernovae; however, more detailed simulations have failed to produce the correct abundances, and so scientists are looking elsewhere. Two promising sites so far are merging neutron stars or the winds off neutron stars, but it’s still an area of active research.
Making the analogue of the r process work for protons as well as neutrons seems like it should be easy. However, unlike neutrons, protons are charged, and the addition process quickly runs up against a Coulomb barrier, caused by the protons repelling each other. As such, the origin of the p-nuclei is a lot harder to explain (and they’re a lot rarer in nature). A small amount of those isotopes that don’t stray too far from the valley of beta stability can be made by capture of a few protons onto seed nuclei, called the p process, but the more extreme nuclei are as of yet not fully explained. Our best candidate for them is the gamma or photodisintegration process. The gamma process happens explosively, when the shockwave from a core-collapse supernova ripples through the neon and oxygen shells surrounding the core and briefly spikes their temperature, density, and pressure. This process essentially takes existing s process elements with roughly equal numbers of protons and neutrons and “melts them down” via photodisintegration, boiling away neutrons to leave proton-rich nuclei.
There are still some problems with the gamma process; for instance, it drastically underproduces the isotope molybdenum-92, which stubbornly refuses to be made in any great quantity by any known nucleosynthetic process. There are a few other options, such as a true rapid proton addition process called rp that takes place at very high temperatures and free proton densities, but the question of the p-nuclei is still open.
So, looking around my dining room right now, I can see the hammer that my housemate was using to put up Christmas decorations. It’s hardened steel – iron and carbon, plus another iron-peak element like chromium for strength – with a rubber grip handle – carbon, hydrogen, oxygen, and some heavier elements in the dyes. So that’s the core of a dying star plus the ashes of the original Big Bang. Not bad. Dying stars aren’t even the most exotic places where the elements are manufactured – as I mentioned, some think the r process is primarily produced in merging neutron stars, and some isotopes must be manufactured in places like X-ray bursts, or a nova flare on a white dwarf, or in the gigantic thermonuclear detonations of type Ia supernovae. The story of where everything came from is a strange one indeed.
And the toothpaste I mentioned at the beginning? Well, it turns out that certain isotopes located in odd little niches of the periodic table can get skipped for one reason or another; examples include lanthanum, tantalum, and, way down at the beginning, fluorine. These elements are made through a process known as neutrino spallation, or the neutrino process.

Forged in the heart of a dying star.
Spallation, which means “to flake,” occurs when a high-energy neutrino strikes a nucleus and knocks out a nucleon via the neutral current interaction; in the case of fluorine, a neutrino hits a neon-20 nucleus and flakes off a proton, leaving behind fluorine-19. Since neutrinos are shy and reluctant to interact with matter, neutrino spallation requires a high energy, high intensity neutrino wind illuminating a large quantity of enriched material. These conditions are achieved just before the shockwave rushes out of the center of a core-collapse supernova, when the dying core emits a brief, extremely intense pulse of neutrinos (the same pulse we detected from Supernova 1987A). As the neutrino pulse passes through the overlying star, it creates spallation elements along the way, including fluorine. Then the shockwave follows, blowing away the outer layers of the star into the interstellar medium, where the liberated fluorine can mix with gas and form into clouds, then protostellar cores, then stellar disks, then planets, then rocks, where some enterprising individual may pick them up and grind them down and mix them in with hydrated silica to form – your morning toothpaste.
Enjoy.


Thanx for this article, great to read. Remarkable in this light of nucleosynthesis, toothpaste and supernovae is another classic article, dating from 1966:
http://adsabs.harvard.edu/full/1966ApJ…143..626C
Who were the authors of this great work? Richard White and Stirling… Colgate! Yep, what’s in a name.